Photometry & Reflectometry
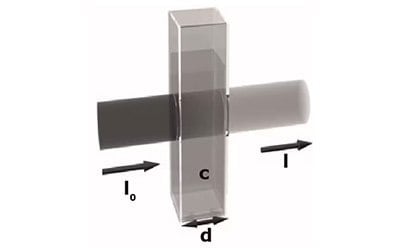
Photometry is the measurement of light absorbed in the ultraviolet (UV) to visible (VIS) to infra-red (IR) range. This measurement is used to determine the amount of an analyte in a solution or liquid. Photometers utilize a specific light source and detectors that convert light passed through a sample solution into a proportional electric signal. These detectors may be for example photodiodes, photoresistors, or photomultipliers. Photometry uses Beer–Lambert’s law to calculate coefficients obtained from the transmittance measurement. A correlation between absorbance and analyte concentration is then established by a test-specific calibration function to achieve highly accurate measurements.
Featured Categories
Spectroquant® instruments, software, accessories, and ready-to-use test kits through innovative solutions and start-to-finish quality assurance ensure precise and rapid results with the easy handling of the system.
Colorimetric and titrimetric test kits with brilliant color cards allow easy and precise analysis for aquaculture, surface water and industrial water testing.
Our selection of MQuant® and Reflectoquant® test strips includes a variety of test strips for fast, precise, and low-cost analysis in-the-field or on-site.
MQuant® and Reflectoquant® pH indicator papers, kits, and non-bleeding pH strips make rapid pH measurement easier than ever.
Photometry
Photometry is a widely used quantitative analysis in research laboratories to determine the amounts of inorganic and organic compounds in solutions and other liquids. Photometry also has broad industrial applications in the determination of contaminants in drinking and wastewater, analysis of nutrients in soil, food and beverage samples, building material composition, and many other areas.
Construction and Working of Photometer
The components of a typical photometer include a light source, monochromator, sample, and detector. Light sources can be tungsten-halogen lamps (generally the source of light used for analyses in the visible light range) or LEDs. For measurements in the UV–Visible range a xenon flash lamp may be used. The monochromator filters the light radiated by the light source to allow only a very narrow spectrum to pass. The light then passes through the cuvette or the sample holding cell. Based on the amount of analyte (or a dye derived from it) that is present in the sample solution, some part of the light is absorbed by the solution and the remaining is transmitted. The transmitted light is directed towards the detectors, which produce an electric current proportional to the light intensity.
Beer–Lambert Law
Beer–Lambert law, also known as Beer’s law, states that the quantity of light absorbed by a sample is directly proportional to the concentration of the analyte present and path length of the light through the sample. “Sample” in this context means either the analyte itself (direct measurement) or a dye derived from the analyte (when using reagents or kits). The relationship is described by the formula:
A = elc |
where |
A = absorbance of the sample |
The spectrophotometer measures the intensity of light before and after passing through a solution and relates it to the transmittance (T) by the following equation: |
Transmittance(T)=It/Io |
It and Io are the intensity of light after and before passing through the solution, respectively. The transmittance is related to the absorbance by the equation: |
Absorbance(A)=−log(T) |
Reflectometry
Reflectometry (also known as remission photometry) is a non-destructive analytical technique that uses the reflection of light by surfaces and interfaces to measure characteristics such as color intensity, film thickness and refractive index.
As with other photometers, the main elements of reflectometers include a light source, usually long-life LEDs of specific wavelengths that are focused onto a sample surface via a lens system and the reflected light is measured by detectors.
Reflectometers are often designed to measure physical characteristics of surfaces like color changes on a test strip. In this approach, a sample can be placed on a test strip and its remission (REM) value can be compared against appropriate controls and standards. As in photometry, the difference in intensity between emitted and reflected light allows a quantitative determination of the concentration of specific analytes.
Reflectometry is commonly used in industrial applications as a rapid, sensitive method for quantitating a wide variety of organic and inorganic parameters in water, food, beverages, and environmental samples as well as other diverse uses such as surface analysis of building materials and skin color quantification.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- A transmittance to absorbance table enables fast conversion from transmittance values to absorbance in the lab or in the field.
- We are a global leader in the life science industry and have produced test kits to measure numerous analytes. Water Online spoke with us about advances in measuring chemical oxygen demand.
- Step-by-step accurate reflectometric determination of ammonium in wastewater with Nessler’s reagent or Indophenol blue using Reflectroquant®system and test strips.
- Preparation of a standard solution for COD/chloride
- Optical density measurement of bacterial cells at 600 nm using pre-programmed method in Spectroquant® Prove spectrophotometers.
- See All (30)
Related Protocols
- Preparation of a standard solution for Hydrogen peroxide
- Spectrophotometric Griess assay protocol for quantitative nitrite analysis in dried meat after extraction with potassium hydrogen phthalate.
- Photometric determination with Chromazurole S subsequent to acid mineralisation; DIN decomposition
- Photometric determination with Chromazurole S subsequent to acid mineralisation
- Photometric determination with Chromazurole S subsequent to fusion melting
- See All (62)
Find More Articles and Protocols
Related Webinars
Join our webinar on June 15th to find out how to quickly analyze chemical disinfectant parameters to ensure the safety of your production line after disinfection & the different methods available.
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email [email protected]
or call +1 (800) 244-1173
Additional Support
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?
























