Selectfluor™
Selectfluor™ (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate), or F-TEDA) is a user-friendly, mild, air- and moisture-stable, non-volatile reagent for electrophilic fluorination. Selectfluor™ reagent is capable of introducing fluorine into organic substrates in one step, with a remarkably broad scope of reactivity.1 Many of these reactions display excellent regioselectivity.
The synthesis of a potent and noncytotoxic nucleoside inhibitor of Hepatitis C virus RNA replication was accomplished using Selectfluor™ Reagent (Scheme 1). This ribonucleoside shows a significantly improved enzymatic stability profile compared to the parent 2’-C-methyladenosine.2
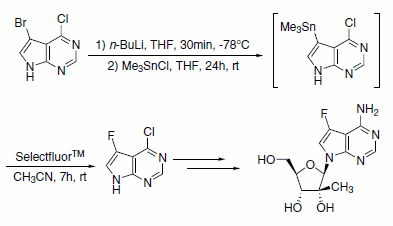
Scheme 1.
Allylic fluorides can be prepared via a sequential cross-metathesis/ electrophilic fluorodesilylation route (Scheme 2). This route avoids the formation of by-products that result from allylic transposition, which is observed when nucleophilic displacement or ring-opening reaction with DAST is attempted.3
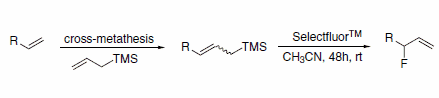
Scheme 2.
Selectfluor™ reagent is also capable of effecting other transformations. One example uses Selectfluor™ reagent as an alternative to silver or mercury salts in the rearrangement of iodides to alcohols (Scheme 3). In the case of an iodochloride (X=Cl), substitution only occurs at the iodide and not the chloride.4
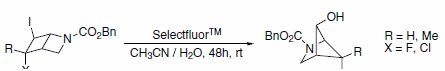
Scheme 3.
Selectfluor™ reagent has been used as an efficient catalyst for the preparation of b-hydroxy thiocyanates using ammonium thiocyanate under mild conditions (Scheme 4). In all cases, the reactions proceed rapidly at room temperature without the formation of the corresponding thiiranes, which are generally the exclusive products when Lewis acids such as Sc(OTf)3 and InCl3 are used.5
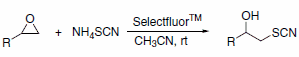
Scheme 4.
References
To continue reading please sign in or create an account.
Don't Have An Account?