Select a Size
About This Item
HPLC: suitable
Skip To
Product Name
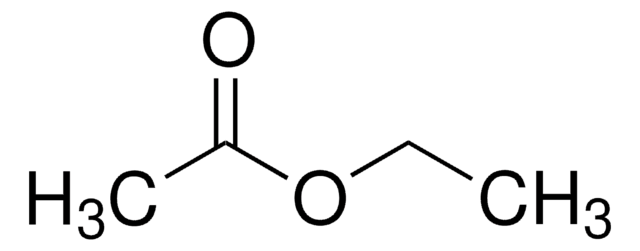
Ethyl acetate, suitable for HPLC, ≥99.8%
InChI key
XEKOWRVHYACXOJ-UHFFFAOYSA-N
InChI
1S/C4H8O2/c1-3-6-4(2)5/h3H2,1-2H3
SMILES string
CCOC(C)=O
grade
HPLC grade
vapor density
3 (20 °C, vs air)
vapor pressure
73 mmHg ( 20 °C)
assay
≥99.8%
form
liquid
autoignition temp.
801 °F
expl. lim.
2.2-11.5 %, 38 °F
technique(s)
GC/MS: suitable
HPLC: suitable
impurities
<0.050% water
evapn. residue
<0.0003%
refractive index
n20/D 1.3720 (lit.)
bp
76.5-77.5 °C (lit.)
mp
−84 °C (lit.)
solubility
alcohol: soluble(lit.)
chloroform: soluble(lit.)
density
0.902 g/mL at 25 °C (lit.)
λ
H2O reference
UV absorption
λ: 254 nm Amax: 1.00
λ: 263 nm Amax: 0.05
λ: 275-400 nm Amax: 0.01
application(s)
food and beverages
format
neat
Quality Level
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | 650528 | 537446 | W241407 |
|---|---|---|---|
| technique(s) GC/MS: suitable, HPLC: suitable | technique(s) HPLC: suitable, gas chromatography (GC): suitable | technique(s) - | technique(s) - |
| assay ≥99.8% | assay 99.9% | assay ≥99.8% | assay ≥99% |
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 400 |
| application(s) food and beverages | application(s) - | application(s) - | application(s) flavors and fragrances |
| solubility alcohol: soluble(lit.), chloroform: soluble(lit.) | solubility alcohol: soluble(lit.), chloroform: soluble(lit.) | solubility alcohol: soluble(lit.), chloroform: soluble(lit.), water: soluble | solubility water: soluble |
| form liquid | form liquid | form liquid | form liquid |
Application
- As solvent for the isolation of Rose hip (Rosa canina L., Rosaceae) powder, via sonication.[1]
- As solvent for the abstraction of volatile thiols from wine for their quantitative estimation by gas chromatography/mass spectrometry (GC-MS).[2]
- Preparation of thin films of TiO2 (titanium dioxide) on glass.[3]
- As an extraction medium in the multi-residue analysis of pesticide residues in fruit and vegetables.[4]
- Acetylation of primary amines to form amides in the presence of dimethyltin(IV) acetic acid distannoxane.[5]
General description
Packaging
As a global leader in lab reagents, we are constantly looking for new ways to optimize the safety of our products. The newly developed 4L solvent bottle design features advanced sealing technology that eliminates leaks to make the handling of solvents safer and more convenient than ever before.
See all the new features here!
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
target_organs
Central nervous system
supp_hazards
Storage Class
3 - Flammable liquids
wgk
WGK 1
flash_point_f
24.8 °F - closed cup
flash_point_c
-4 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service