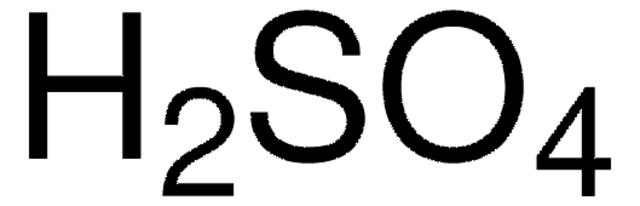The boiling point of this product is listed in Section 9 on the Safety Data Sheet as 554 °F / 290 °C. The melting point is listed as 50.56 °F / 10.31 °C.
Select a Size
About This Item
Skip To
grade
ACS reagent
Quality Level
Agency
suitable for EPA 1613
suitable for SM 4500 - NH3
suitable for SM 5210
vapor density
<0.3 (25 °C, vs air)
vapor pressure
1 mmHg ( 146 °C)
description
Free from suspended or insoluble matter
Nominally 95-98% H2SO4
Assay
95.0-98.0%
form
liquid
ign. residue
≤5 ppm
color
APHA: ≤10
pH
1.2 (5 g/L)
bp
~290 °C (lit.)
solubility
water: soluble
density
1.840 g/mL at 25 °C (lit.)
anion traces
MnO4- reducers: ≤2 ppm
chloride (Cl-): ≤0.2 ppm
nitrate (NO3-): ≤0.5 ppm
cation traces
As: ≤0.01 ppm
Fe: ≤0.2 ppm
Hg: ≤5 ppb
NH4+: ≤2 ppm
heavy metals: ≤1 ppm (by ICP)
application(s)
PFAS testing
SMILES string
OS(O)(=O)=O
InChI
1S/H2O4S/c1-5(2,3)4/h(H2,1,2,3,4)
InChI key
QAOWNCQODCNURD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
1 of 3
This Item | 07208 | 84727 |
|---|---|---|
| grade ACS reagent | grade - | grade for inorganic trace analysis |
| assay 95.0-98.0% | assay 95-97% | assay ≥97.0% |
| Quality Level 200 | Quality Level 200 | Quality Level 100 |
| agency suitable for EPA 1613, suitable for SM 5210, suitable for SM 4500 - NH3 | agency - | agency suitable for SM 4500 - NH3 |
| form liquid | form liquid | form liquid |
| pH 1.2 (5 g/L) | pH - | pH - |
Application
- In the hydrolysis of primary or secondary nitroalkane salts to aldehydes or ketones.
- In the selective hydrolysis of nitriles to amides.
- To synthesize amides of t-alkylcarbinamines via Ritter reaction between nitriles and alkenes.
- To prepare 3,4-dialkyl-2(5H)-furanones by carbonylation of α,β-unsaturated aldehydes.
- In the nitration of aromatic carbocycles and heteroaromatic compounds.
- In the Friedel–Craft ketone synthesis using anhydrides via α-amidoalkylation reaction.
H2SO4 can also be used in the following reactions:
- Michael′s addition reactions
- Electrophilic substitution reactions
- Esterification of highly hindered aromatic acids
- Aldol condensation reaction
- Beckmann rearrangement
- Hofmann–Loffler–Freytag reaction
- Isomerization
- Dehydrogenation
- Sulfonation
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Simple to use laboratory reference chart for scientists, researchers and lab technicians.
High-Performance Thin-Layer chromatography (HPTLC) quantification of methylglyoxal (MGO) in complex and matrix rich manuka honey offering quick sample preparation, high-matrix tolerance, and high-throughput.
Biểu đồ axit và bazơ liệt kê độ mạnh của axit và bazơ (mạnh nhất đến yếu nhất) theo thứ tự. Biểu đồ tham chiếu phòng thí nghiệm dễ sử dụng cho các nhà khoa học, nhà nghiên cứu và kỹ thuật viên phòng thí nghiệm.
Sắc ký lớp mỏng hiệu suất cao (HPTLC) Định lượng methylglyoxal (MGO) trong mật ong manuka phức tạp và ma trận cung cấp chế biến mẫu nhanh, dung sai ma trận cao và thông lượng cao.
Protocols
To measure 5′-nucleotidase activity, this procedure uses adenosine 5’-monophosphate and a color reagent to create a standard curve for determining the micromoles of phosphorus liberated.
Derived from procedure SSPHYT02. Includes template updates to current SOP specifications and incorporation of notes into the procedure.
Lấy từ quy trình SSPHYT02. Bao gồm các cập nhật mẫu đối với các thông số kỹ thuật SOP hiện tại và kết hợp các ghi chú vào quy trình.
Related Content
This page is intended to make it easier to find the consumables you need based on the analytical method you’re using. Methods included on this page come from the EPA, Standard Methods and ASTM.
-
Boiling point of sulfuric acid
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/418/501/product-dating-information-06-25-mk.pdfHelpful?
-
-
Is the range of concentration listed have to do with volume or will an exact percentage be available on the bottle when received for the purposes of further dilution.
1 answer-
The specification for this product (95.0 to 98.0 percent) is for the weight percent of the sulfuric acid; the remaining 2 to 5 percent by weight is water. The lot-specific weight percent is reported as the Purity by Sodium Hydroxide (NaOH) titration on the certificate of analysis for each lot. The result is not printed on the bottle. Practically speaking, whether the percentage is 95.0 percent or 98.0 percent or somewhere in between, the material would be handled the same way.
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
Difference between 258105-500ML, and 258105-500ML-PC, Mainly the PC part of the partnumber.
1 answer-
Product 258105-500ML is packaged in a poly bottle, The package sizes listing the -PC suffix, including 258105-500ML-PC, are packaged in PVC-coated glass bottles.
Helpful?
-
-
What is the concentration of Sulfuric acid, Product 258105, in moles per liter (M)?
1 answer-
This material has a calculated, theoretical concentration of 18 M.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
How was the concentration of Sulfuric acid, Product 258105, calculated?
1 answer-
The molarity is calculated using the following equation:molarity=[(1000 X Density)/molecular weight] X (percent purity/100).
Helpful?
-
-
What is Sulfuric acid, Product 258105, dissolved in?
1 answer-
This material is not dissolved in anything. Rather it is pure sulfuric acid, which is a liquid at room temperature.
Helpful?
-
-
How is Sulfuric acid, Product 258105, to be stored?
1 answer-
This material is a room temperature storage item.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service