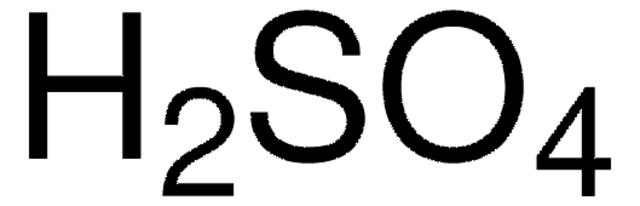If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/418/501/product-dating-information-06-25-mk.pdf
Sélectionner une taille de conditionnement
188,00 $
651,00 $
2 130,00 $
A propos de cet article
Passer à
Nom du produit
Acide sulfurique, 99.999%
InChI key
QAOWNCQODCNURD-UHFFFAOYSA-N
InChI
1S/H2O4S/c1-5(2,3)4/h(H2,1,2,3,4)
SMILES string
OS(O)(=O)=O
agency
suitable for SM 5210
vapor density
<0.3 (25 °C, vs air)
vapor pressure
1 mmHg ( 146 °C)
description
Nominally 95-98% H2SO4
assay
99.999%
form
viscous liquid
color
clear
pH
1.2 (5 g/L)
bp
~290 °C (lit.)
solubility
water and organic: soluble
density
1.840 g/mL at 25 °C (lit.)
Quality Level
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
1 of 2
Cet article | 1.00713 |
|---|---|
| assay 99.999% | assay 95-97% (alkalimetric) |
| Quality Level 200 | Quality Level 500 |
| agency suitable for SM 5210 | agency BP, JPE, NF, Ph. Eur., suitable for EPA 1613 |
| form viscous liquid | form liquid |
| solubility water and organic: soluble | solubility - |
| pH 1.2 (5 g/L) | pH 0.3 (25 °C, 49 g/L in H2O) |
Analysis Note
Application
- comme catalyseur dans l'estérification des acides acétique et palmitique. [1]
- comme solvant dans la synthèse de sulfure de technétium binaire par réaction entre du pertechnétate et du sulfure d'hydrogène. [2]
- comme desséchant, comme catalyseur et comme réactif dans l'industrie chimique[3][4].
- dans la préparation d'un composite silice/acide sulfurique servant de catalyseur en synthèse organique.[5]
General description
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Classe de stockage
8B - Non-combustible corrosive hazardous materials
wgk
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Simple to use laboratory reference chart for scientists, researchers and lab technicians.
Contenu apparenté
This page is intended to make it easier to find the consumables you need based on the analytical method you’re using. Methods included on this page come from the EPA, Standard Methods and ASTM.
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
What is the molarity of lot MKCS5290 for product 339741?
1 answer-
The molarity of lot MKCS5290 for product 339741 is 18.76M.
Helpful?
-
-
What is the molarity or normality for Product 339741?
1 answer-
The molarity of Product 339741 is 18.74 M, which means the normality is 37.4 since it is a diprotic acid.
Helpful?
-
-
what mineral is used to make sulfonic acid
1 answer-
Product number 339741 represents sulfuric acid. The only 'mineral' present is sulfur. Any compounds used in the production or purification of this product would be considered proprietary. To address the question in reference to 'Sulfonic acid', this is a functional group and does not occur naturally. Therefore, no additional information can be provided.
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique