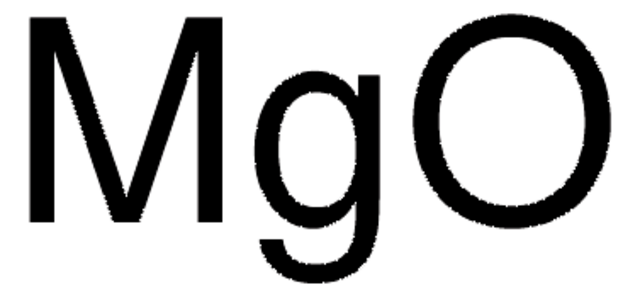Select a Size
₹3,998.45
₹6,266.70
About This Item
powder
Skip To
Assay
98%
form
beads or granules (May Contain Dark Specs)
powder
impurities
<5% adsorbed gases removable by calcining at >900°C.
particle size
-10-+50 mesh
mp
2852 °C (lit.)
SMILES string
O=[Mg]
InChI
1S/Mg.O
InChI key
CPLXHLVBOLITMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | 342793 | 307742 | 1.05866 |
|---|---|---|---|
| form beads or granules (May Contain Dark Specs), powder | form powder | form powder | form solid |
| assay 98% | assay ≥99% trace metals basis | assay - | assay ≥97% (complexometric) |
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 300 |
| impurities <5% adsorbed gases removable by calcining at >900°C. | impurities - | impurities - | impurities ≤0.02% Substances insoluble in hydrochloric acid, ≤0.02% Substances precipitated by ammonia, ≤0.4% Substances soluble in water |
| mp 2852 °C (lit.) | mp 2852 °C (lit.) | mp 2852 °C (lit.) | mp 2800 °C |
| particle size -10-+50 mesh | particle size -325 mesh | particle size - | particle size - |
Application
- Bio-corrosion behavior and mechanical characteristics of magnesium-titania-hydroxyapatite nanocomposites coated by magnesium-oxide flakes and silicon for use as resorbable bone fixation material.: Investigates magnesium oxide coatings to improve the biocompatibility and mechanical properties of bone implants, crucial in analytical and biomaterials chemistry (Khalajabadi et al., 2018).
- Certification of caffeine reference material purity by ultraviolet/visible spectrophotometry and high-performance liquid chromatography with diode-array detection as two independent analytical methods.: While focused on caffeine, the rigorous analytical methods described could be applicable for studies involving magnesium oxide in chemical analysis (Shehata et al., 2016).
- Hybrid organic-inorganic coatings via electron transfer behaviour.: Explores the electron transfer properties of coatings involving inorganic compounds like magnesium oxide, important for materials science and analytical chemistry (Zoubi et al., 2017).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service