This product may be stored at room temperature. For products with no specified storage temperature given on the label or product page, ambient storage may be assumed. Products that require controlled temperature storage, such as refrigeration or freezer conditions, storage temperatures are published on the product label, Certificate of Analysis, and the Safety Data Sheet (SDS).
Select a Size
₪298.00
₪598.00
₪904.00
₪1,315.00
About This Item
Skip To
Product Name
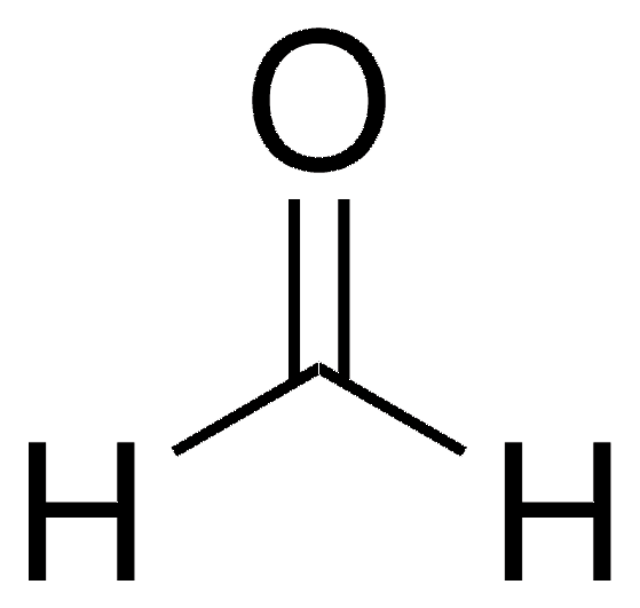
Formaldehyde solution, meets analytical specification of USP, ≥34.5 wt. %
SMILES string
[H]C([H])=O
InChI
1S/CH2O/c1-2/h1H2
InChI key
WSFSSNUMVMOOMR-UHFFFAOYSA-N
vapor density
1.03 (vs air)
vapor pressure
52 mmHg ( 37 °C)
autoignition temp.
572 °F
quality
meets analytical specification of USP
contains
9.0-15.0% methanol as stabilizer (GC)
concentration
≥34.5 wt. %
25-50%
impurities
acidic reac. substances, complies
residual solvents, complies
ign. residue
≤0.01% (as SO4)
density
1.09 g/mL at 25 °C (lit.)
suitability
corresponds for identity
Quality Level
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 47608 | 252549 | F1635 |
|---|---|---|---|
| Quality Level 200 | Quality Level 100 | Quality Level 200 | Quality Level 200 |
| density 1.09 g/mL at 25 °C (lit.) | density 1.09 g/mL at 20 °C, 1.09 g/mL at 25 °C (lit.) | density 1.09 g/mL at 25 °C (lit.) | density 1.09 g/mL at 25 °C (lit.) |
| vapor density 1.03 (vs air) | vapor density 1.03 (vs air) | vapor density 1.03 (vs air) | vapor density 1.03 (vs air) |
| vapor pressure 52 mmHg ( 37 °C) | vapor pressure 52 mmHg ( 37 °C) | vapor pressure 52 mmHg ( 37 °C) | vapor pressure 52 mmHg ( 37 °C) |
| autoignition temp. 572 °F | autoignition temp. 572 °F | autoignition temp. 572 °F | autoignition temp. 572 °F |
| quality meets analytical specification of USP | quality - | quality - | quality - |
Application
General description
Other Notes
The article number 15512-6X1L-R will be discontinued. Please order the single bottle 15512-1L-R which is physically identical with the same exact specifications.
signalword
Danger
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Carc. 1B - Eye Dam. 1 - Muta. 2 - Skin Corr. 1B - Skin Sens. 1 - STOT SE 1 - STOT SE 3
target_organs
Eyes,Central nervous system, Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk
WGK 3
flash_point_f
143.6 °F - closed cup
flash_point_c
62 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
-
What is the storage conditions of Formaldehyde solution 15512-1L-R STBL0225
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service