About This Item
Skip To
Product Name
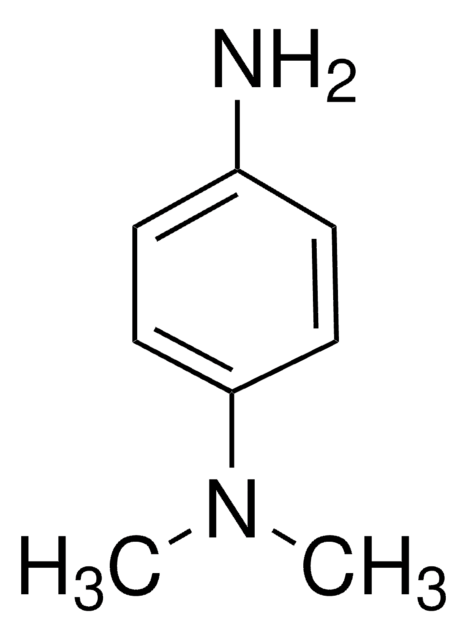
4-(2,3-Dihydro-1,3-dimethyl-1H-benzimidazol-2-yl)-N,N-dimethylbenzenamine, 97% (HPLC)
InChI
1S/C17H21N3/c1-18(2)14-11-9-13(10-12-14)17-19(3)15-7-5-6-8-16(15)20(17)4/h5-12,17H,1-4H3
SMILES string
CN(C)c1ccc(cc1)C2N(C)c3ccccc3N2C
InChI key
AKIIMLCQTGCWQQ-UHFFFAOYSA-N
assay
97% (HPLC)
form
solid
mp
105-110 °C
Quality Level
Related Categories
1 of 4
This Item | 185582 | 193992 | 549983 |
|---|---|---|---|
| assay 97% (HPLC) | assay ≥99% | assay 97% | assay ≥99% |
| Quality Level 100 | Quality Level 200 | Quality Level 200 | Quality Level 100 |
| mp 105-110 °C | mp −75 °C (lit.) | mp 34-36 °C (lit.) | mp - |
| form solid | form liquid | form solid | form - |
Application
General description
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
溶液プロセスにより作製される低分子太陽電池の最近の成果についてご紹介します。低分子ドナー化合物の独創的な分子設計により、電力変換効率は約8%に向上しています。
溶液処理塗布した後、高品質のペンタセン膜に熱的に変換することが可能な「可溶性ペンタセン前駆体」についてご紹介します。
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service