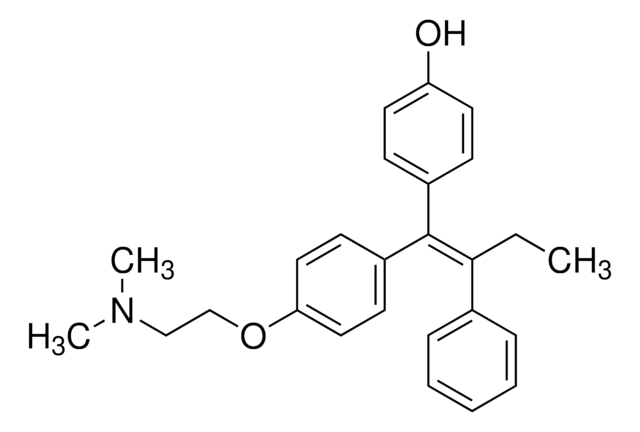
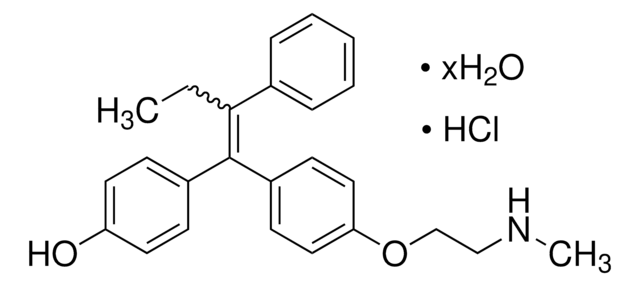
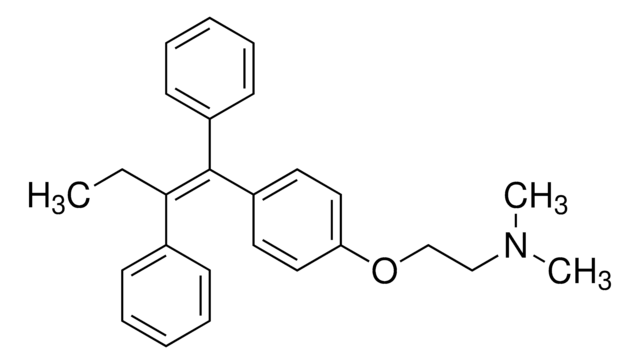
4-Hydroxytamoxifen (4-OHT) is a metabolite of the antiestrogen tamoxifen in humans and other mammals.
[4] Both the Z (
trans) and E (
cis) 4-OHT isomers exhibit antiestrogenic properties in immature rats. Studies on the structure-function relationships of fixed ring systems have revealed that the trans isomer is a potent antiestrogen, while the cis isomer is relatively weaker (about 100 times less potent) as an antiestrogen in T47D breast cancer cells
in vitro. 4-OHT binds to estrogen receptors (ER) and estrogen-related receptors (ERR), exerting both estrogenic and anti-estrogenic effects. This compound is a cell-permeable, selective estrogen receptor modulator (SERM). Compared to tamoxifen and its other metabolites, 4-OHT demonstrates a higher affinity for binding to estrogen receptors, resulting in 50 to 100-fold greater potency in inhibiting cell proliferation in normal human breast cells and breast cancer cell lines in culture.
[5] Moreover, 4-OHT has been found effective in inhibiting the growth of these cells in the absence of estrogen when cell proliferation was induced by insulin or epidermal growth factor.