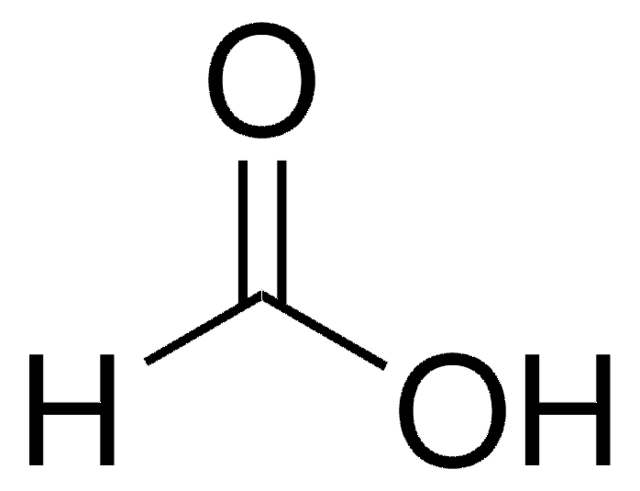
The product is titrated against standardized NaOH. The lot-specific results that is reported on its Certificate of Analysis is determined as % w/w.
Select a Size
About This Item
Skip To
grade
reagent grade
Quality Level
vapor density
1.6 (vs air)
vapor pressure
44.8 mmHg ( 20 °C)
Assay
≥95%
form
liquid
autoignition temp.
1004 °F
contains
<2.5% water as stabilizer
expl. lim.
57 %
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤1% acetic acid
refractive index
n20/D 1.370 (lit.)
pH
2.2 (20 °C, 2.2 g/L)
bp
100-101 °C (lit.)
mp
8.2-8.4 °C (lit.)
solubility
water: miscible
density
1.22 g/mL at 25 °C (lit.)
greener alternative category
storage temp.
room temp
SMILES string
OC=O
InChI
1S/CH2O2/c2-1-3/h1H,(H,2,3)
InChI key
BDAGIHXWWSANSR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 33015-M | W248703 | 1.00263 |
|---|---|---|---|
| grade reagent grade | grade ACS reagent, puriss. p.a. | grade FG, Halal, Kosher | grade - |
| assay ≥95% | assay ≥98% | assay ≥95% | assay ≥98% (alkalimetric) |
| Quality Level 100 | Quality Level 100 | Quality Level 400 | Quality Level 500 |
| form liquid | form liquid | form liquid | form liquid |
| pH 2.2 (20 °C, 2.2 g/L) | pH 2.2 (20 °C, 2.2 g/L) | pH 2.2 (20 °C, 2.2 g/L) | pH 2.2 (20 °C, 10 g/L in H2O) |
| storage temp. room temp | storage temp. - | storage temp. - | storage temp. 15-25°C |
General description
Application
- Electrochemical CO(2) Reduction on Metallic and Oxidized Tin: This study uses grand-canonical density functional theory (DFT) and in situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy (ATR-SEIRA) to investigate electrochemical carbon dioxide reduction on tin surfaces, where formic acid could play a role in understanding reaction mechanisms (Whittaker et al., 2024).
- Simultaneous Measurement of COVID-19 Treatment Drugs: This research demonstrates the use of UPLC-MS/MS for the simultaneous measurement of COVID-19 treatment drugs in rat plasma, indicating the importance of formic acid in preparing samples or as a mobile phase additive for better chromatographic separation (Zhou et al., 2024).
- Metabolite Profiling of Liquiritin: The study involves metabolite profiling using ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS), where formic acid is likely utilized in sample preparation or chromatographic processes (Chen et al., 2024).
- Analysis of Cocaine and Its Metabolites: This article explores solid-phase extraction followed by UHPLC-ESI-MS/MS analysis of cocaine metabolites, a method that often incorporates formic acid to enhance the ionization of analytes (Makhdoom et al., 2024).
- Determination of Antimicrobial Compounds in Pigs: This research uses UHPLC-MS/MS for the simultaneous determination of various antimicrobial compounds, demonstrating formic acid′s role in sample processing and chromatographic separation (Nowacka-Kozak et al., 2024).
- synthesis of graphene from graphene oxide. [2]
- catalytic reduction of chromium (Cr(VI) to Cr(III)) by colloidal palladium. [3]
Additionally, it is used as a hydrogen donor during the transformation of the furanose form into the pyranose form of glucose and in the catalytic transfer hydrogenation reaction. [5]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
121.1 °F - closed cup
Flash Point(C)
49.5 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
LC-MS/MS-based determination of 19 sulfonamides, 13 quinolones, and 3 tetracycline drug residues in pork meat samples using Supel™ Swift HLB for extraction.
-
Is the assay value expressed in %w/v or %w/w, please?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/418/501/product-dating-information-06-25-mk.pdfHelpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service