Determination of 11 β-Agonist Residues in Pork by LC-MS in relation to GB/T 22286-2008 using a Pentafluorophenyl (PFP) HPLC Phase
Dean Duan, Senior Scientist
Merck R&D APAC Lab, Shanghai, China
Abstract
The GB/T 22286-2008 describes for the determination of β-agonist residues in food of animal origin a C18 column for the LC-MS. In this study for improved separation, Ascentis® Express pentafluorophenyl phase (PFP) HPLC column with 2.7µm particles, was used to determine the β-agonists clenbuterol, terbutaline, ractopamine, cimbuterol, isoxsuprine hydrochloride, salbutamol, bromchlorbuterol hydrochloride, cimaterol, brombuterol, mabuterol and mapenterol in pork samples by LC-MS/MS after SPE cleanup with a mixed mode phase. The separation was performed in under 8 min. The LOD for the 11 β-receptor agonists ranged from 0.01 to 0.06 µg/kg and the LOQ is from 0.03 to 0.18 µg/kg. The % recovery ranged from 70 to 115%.
Read more:
Introduction
β-Receptor agonists are used in livestock to promote “lean muscle” as opposed to fat build up. However, there is evidence to indicate that the intake of meat with β-receptor agonist residues can have adverse effects on human health.1 There are also environmental concerns as such residues can leach into the soil and waterways.2 Therefore the use of β-agonists has been prohibited in many countries.
The Chinese national standard GB/T 22286-20083 for β-agonists residues in foodstuff of animal origin describes an LC-MS method using a C18 column (150 mm length x 2.1 mm internal diameter, 5 µm particle size). However, when this dimension column was used to separate the 11 β-receptor agonists, some peaks were very broad. For that reason, an alternative column phase and dimension was considered. In this study, a superficially porous particle (SPP)/Fused-Core® column with a pentafluorophenyl phase (PFP, USP L43), the Ascentis® Express F5 column (100 x 2.1 mm, 2.7 µm) is used to perform the separation. The F5 was selected to provide orthogonal selectivity to a C18 phase and improved peak shapes. For the SPE cleanup a mixed mode cation exchange phase is recommended by the GB method. Here we evaluated the recovery of the 11 β-receptor agonists using a Discovery® DSC-MCAX SPE tube, which contains both octyl (C8) & benzene sulfonic acid (SCX) functionalities.

Figure 1.Structures of β-receptor agonists determined.
Experimental Conditions
Standard Preparation
- Standard Mixture (1 mg/mL): Weigh 10 mg of each β-agonist into a 10 mL volumetric flask. Add 7 mL methanol and sonicate for 5 minutes. Top to mark with methanol and mix well. This is the standard mixture, 1 mg/mL.
- Working Standard Solution (10 µg/mL): Transfer 100 μL of the standard mixture into a 10 mL volumetric flask. Top to mark with methanol and mix well.
- Internal Standard Mixture (1 mg/mL): Weigh 10 mg of clenbuterol-d9 and salbutamol-d3 into a 10 mL volumetric flask. Add 7 mL methanol and sonicate for 5 minutes. Top to mark with methanol and mix well.
- Working Internal Standard Mixture (10 μg/mL): Transfer 100 μL of internal standard mixture into a 10 mL volumetric flask. Top to mark with methanol and mix well. This is the working internal standard mixture, 10 μg/mL.
- Series of Calibration Standards: 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, and 50 ng/mL with 5 ng/mL clenbuterol- d9 and salbutamol- d3 using 0.1% v/v formic acid in 10% v/v methanol.
Sample Preparation
- Sodium acetate buffer solution (0.2 mol/L): Weigh 13.6 g of sodium acetate into a 500 mL volumetric flask. Add ~400 mL of water and adjust to pH 5.2 with acetic acid.
- Weigh 2 g of homogenized pork meat sample (±0.01 g) into a 50 mL centrifuge tube.
- Add 6 mL of 0.2 mol/L sodium acetate buffer solution and 50 μL of β-glucuronidase/arylsulfatase. Mix well, protect from light, and incubate in a 37 °C water bath for 16 hours.
- Cool to room temperature and add 25 μL of 10 μg/mL working internal standard mixture. Vortex and centrifuge at 8000 rpm for 8 minutes. Retain the supernatant.
- Add 5 mL of 0.1 mol/L perchloric acid solution to the supernatant. Vortex and adjust the pH to 1.0 ± 0.2 with hydrochloric acid.
- Centrifuge at 8000 rpm for 8 minutes and transfer the supernatant to another centrifuge tube. Adjust the pH to 10 ± 0.5 using a 10 mol/L sodium hydroxide solution.
- Add 10 mL of saturated sodium chloride solution and 10 mL of isopropanol-ethyl acetate (6:4 v/v) mixture.
- Centrifuge at 5,000 rpm for 10 minutes.
- Transfer the supernatant to another vial and dry it down with nitrogen gas in a 40 °C water bath.
- Add 5 mL of a 0.2 mol/L sodium acetate solution. Sonicate to dissolve the residue for SPE clean-up. This is the pork sample extract. SPE clean-up conditions are outlined in Table 1.
SPE Conditions | |
|---|---|
Sample/matrix: | Pork sample extract solution |
SPE tube/cartridge: | Discovery® DSC-MCAX, 100 mg/3 mL (52783-U) |
Conditioning: | 3 mL methanol, followed by 3 mL of 2% v/v formic acid aqueous solution |
Sample addition: | Load all the pork extract solution (5 mL) and adjust the flow rate to 1 drop per second |
Washing: | 2 mL of water, 2 mL of 2% v/v formic acid, and then 2 mL methanol |
Elution: | 2 mL of 5% v/v ammonia in methanol |
Eluate post-treatment: | Evaporate to dryness under nitrogen at 40 ℃, reconstitute in 0.5 mL of 0.1% v/v formic acid in 10% v/v methanol |
LC-MS Analysis | ||||
|---|---|---|---|---|
| LC Conditions | ||||
Instrument: | Acquity UPLC I-class plus | |||
Column: | Ascentis® Express F5, 100 x 2.1 mm I.D., 2.7 μm particles (53569-U) | |||
Mobile phase: | [A] 0.1% Formic acid in water; [B] 0.1% formic acid in acetonitrile | |||
Gradient: | Time (min) | A% | B% | |
0 | 95 | 5 | ||
1 | 95 | 5 | ||
5.0 | 5 | 95 | ||
6.5 | 5 | 95 | ||
6.60 | 95 | 5 | ||
8.5 | 95 | 5 | ||
Flow rate: | 0.4 mL/min | |||
Pressure: | 3892 to 4996 psi | |||
Column temp.: | 25℃ | |||
Detector: | MS (see conditions below), MRM (see Table 3) | |||
Injection: | 5 μL | |||
| MS Conditions | ||||
Instrument: | Xevo TQ-S | |||
Polarity: | Positive | |||
Spray voltage: | 3.0 kV (ESI+) | |||
Capillary temp: | 550℃ | |||
Desolvation (L/Hr): | 1000 | |||
Cone (L/Hr): | 150 | |||
Nebuliser (Bar): | 7 | |||
System Suitability
Acceptance Criteria as described in GB/T 22286-2008
- Linear correlation coefficient: R2 > 0.99
- PE recovery rate: 70 - 120%.
- LOD: <0.2 µg/kg; LOQ <0.5 µg/kg
Results & Discussion
Chromatographic results on the used Ascentis® Express F5 column for the 11 β-agonists in a 5 ng/mL standard, a spiked pork meat sample (1.25 µg/kg) and a blank are displayed in the Figures 2-4. The specificity data for the 11 β-agonists – MRM transitions and retention times – are shown in Table 3. The repeatability data of multiple injections of the 5 ng/mL standard is presented in Table 4 and show % RSDs ranging from 1.5 to 4.6. The linearity data for the external calibration for the range of 0.1 to 50 ng/mL and from this the derived equivalent LOD and LOQ values for a pork meat sample are presented in Table 5 showing that this methods sensitivity meets the criteria of the GB method. For all analytes a R2 value of >0.99 was determined (0.9936 to 0.9982). The calibration curve for clenbuterol is displayed in Figure 5 as example. The % recovery for the 11 β-agonists spiked at 1.25 µg/kg in pork meat after SPE cleanup ranged from 70 to 115% (Table 6) meeting the system suitability criteria.
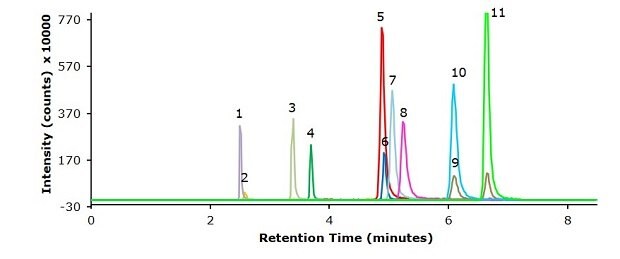
Figure 2.Injection of a standard mixture with 5 ng/mL each of 11 β-agonists (Table 3).
Peak | Compound name | Retention Time (min) | Transition (m/z) | Cone (V) | Colllision (V) | Dwell (s) |
|---|---|---|---|---|---|---|
1 | Salbutamol | 2.52 | 240.2 > 148.0 | 30 | 20 | 0.025 |
2 | Terbutaline | 2.59 | 226.2 > 107.1 | 38 | 30 | 0.025 |
3 | Cimbuterol | 3.40 | 234.2 > 160.0 | 26 | 14 | 0.025 |
4 | Racopamine | 3.70 | 302.3 > 164.1 | 64 | 14 | 0.025 |
5 | Clenbuterol | 4.90 | 277.1 > 203.0 | 26 | 16 | 0.025 |
6 | Isoxsuprine | 4.93 | 302.3 > 150.0 | 64 | 20 | 0.025 |
7 | Bromchlorbuterol | 5.06 | 321.1 > 246.9 | 32 | 16 | 0.025 |
8 | Brombuterol | 5.24 | 365.1 > 290.9 | 32 | 18 | 0.025 |
9 | Cimaterol | 6.10 | 219.0 > 192.0 | 86 | 18 | 0.025 |
10 | Mabuterol | 6.10 | 311.2 > 201.9 | 40 | 32 | 0.025 |
11 | Mapenterol | 6.64 | 325.0 > 237.0 | 40 | 24 | 0.025 |
12 | Clenbuterol-d9 (Internal standard) | 4.90 | 286.3 > 204.0 | 34 | 16 | 0.025 |
13 | Salbutamol-d3 (Internal standard) | 2.52 | 243.2 > 151.1 | 30 | 18 | 0.025 |
*Note: Salbutamol-d3 served as an internal standard (IS) for salbutamol, terbutaline and ractopamine, while clenbuterol- d9 was used as IS for the other compounds. | ||||||

Figure 3.Pork meat sample spiked with standard mixture of 11 β-agonists at 1.25 μg/kg.

Figure 4.Injection of a blank pork meat sample.
Repeatability | ||||||||
|---|---|---|---|---|---|---|---|---|
Compound | Area ratio of analyte to internal standard | Standard Deviation | RSD (%) | |||||
Injection 1 | Injection 2 | Injection 3 | Injection 4 | Injection 5 | Mean | |||
Salbutamol | 1.334 | 1.226 | 1.247 | 1.235 | 1.293 | 1.267 | 0.045 | 3.6 |
Terbutaline | 0.245 | 0.244 | 0.236 | 0.231 | 0.248 | 0.241 | 0.007 | 2.9 |
Cimbuterol | 0.372 | 0.384 | 0.374 | 0.342 | 0.382 | 0.371 | 0.017 | 4.6 |
Racopamine | 1.235 | 1.221 | 1.231 | 1.276 | 1.267 | 1.246 | 0.024 | 1.9 |
Clenbuterol | 0.955 | 0.920 | 0.933 | 0.938 | 0.953 | 0.940 | 0.015 | 1.5 |
Isoxsuprine | 0.202 | 0.191 | 0.195 | 0.191 | 0.197 | 0.195 | 0.005 | 2.4 |
Bromchlorbuterol | 0.667 | 0.660 | 0.679 | 0.676 | 0.691 | 0.675 | 0.012 | 1.8 |
Brombuterol | 0.465 | 0.459 | 0.498 | 0.486 | 0.506 | 0.483 | 0.020 | 4.2 |
Cimaterol | 0.180 | 0.170 | 0.188 | 0.177 | 0.183 | 0.180 | 0.007 | 3.7 |
Mabuterol | 0.865 | 0.875 | 0.923 | 0.935 | 0.947 | 0.909 | 0.037 | 4.0 |
Mapenterol | 1.235 | 1.204 | 1.266 | 1.260 | 1.318 | 1.257 | 0.042 | 3.4 |
Calibration and Method Sensitivity Data
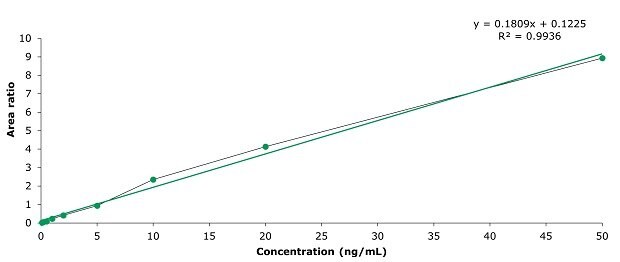
Figure 5.Calibration curve of clenbuterol at 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, and 50 ng/mL.
Compound | Calibration Range (ng/mL) | No. of Calibrators | R2 | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|
Salbutamol | 0.1 – 50 | 9 | 0.9937 | 0.03 | 0.10 |
Terbutaline | 0.9967 | 0.01 | 0.03 | ||
Cimbuterol | 0.9982 | 0.05 | 0.14 | ||
Racopamine | 0.9939 | 0.03 | 0.07 | ||
Clenbuterol | 0.9936 | 0.06 | 0.18 | ||
Isoxsuprine | 0.9943 | 0.04 | 0.10 | ||
Bromchlorbuterol | 0.9964 | 0.05 | 0.14 | ||
Brombuterol | 0.9961 | 0.04 | 0.11 | ||
Cimaterol | 0.9972 | 0.04 | 0.13 | ||
Mabuterol | 0.9966 | 0.05 | 0.15 | ||
Mapenterol | 0.9958 | 0.04 | 0.12 | ||
*The GB method criteria are LOD <0.2 µg/kg and LOQ <0.5 µg/kg | |||||
SPE Recovery Determination | ||||||
|---|---|---|---|---|---|---|
Compound | Sample 1 (%) | Sample 2 (%) | Sample 3 (%) | Mean (%) | Standard Deviation | RSD (%) |
Salbutamol | 77 | 70 | 68 | 71.7 | 4.7 | 6.6 |
Terbutaline | 79 | 92 | 78 | 83.0 | 7.8 | 9.4 |
Cimbuterol | 72 | 80 | 71 | 74.3 | 4.9 | 6.6 |
Ractopamine | 70 | 68 | 72 | 70.0 | 2.0 | 2.9 |
Clenbuterol | 109 | 93 | 97 | 99.7 | 8.3 | 8.4 |
Isoxsuprine | 105 | 88 | 92 | 95.0 | 8.9 | 9.4 |
Bromchlorbuterol | 92 | 83 | 87 | 87.3 | 4.5 | 5.2 |
Brombuterol | 82 | 73 | 74 | 76.3 | 4.9 | 6.5 |
Cimaterol | 112 | 109 | 111 | 110.7 | 1.5 | 1.4 |
Mabuterol | 116 | 116 | 113 | 115.0 | 1.7 | 1.5 |
Mapenterol | 90 | 94 | 93 | 92.3 | 2.1 | 2.3 |
Conclusion
The GB/T 22286-2008 describes for the determination of β-agonist residues in food of animal origin a C18 column for the LC-MS. During method development broad peaks were observed for certain compounds (results not shown), hence an F5 (pentafluorophenylpropyl, PFP) stationary phase was evaluated providing orthogonal selectivity to the C18 phase. For the developed method an Ascentis® Express F5 column with 2.7 µm Fused-Core® particles was used to separate and quantify the β-agonists in under 8 min showing improved peak shapes. For the sample preparation with a mixed mode cationic SPE phase, the Discovery® MCAX with C8 & SCX functionalities was applied. The developed method overall provided good recoveries, precision, accuracy, and sensitivity. It met or exceeded the suitability requirements of the current Chinese GB method (GB/T 22286-2008).
The used Ascentis® Express F5 HPLC column can therefore be a suitable and efficient alternative to the described C18 column for the determination of β-agonists in meat samples.
See more applications for Food & Beverage testing.
References
To continue reading please sign in or create an account.
Don't Have An Account?