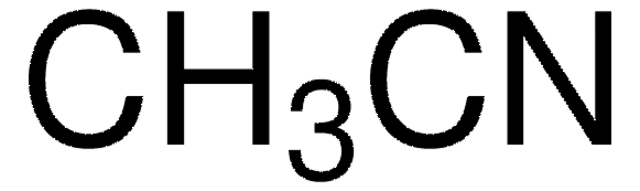Scegli un formato
Informazioni su questo articolo
Vai a
Nome del prodotto
Acetonitrile, EMPLURA®, for preparative purposes
Grado
synthesis grade
Densità del vapore
1.41 (vs air)
Tensione di vapore
72.8 mmHg ( 20 °C)
97 hPa ( 20 °C)
Descrizione
for analytical purposes
for extraction
Nome Commerciale
EMPLURA®
Saggio
≥90% (HPLC)
Stato
liquid
Temp. autoaccensione
524 °C
973 °F
Limite di esplosione
16 %
Produttore/marchio commerciale
Calbiochem®
dilution
(low-cost alternative, for basic applications)
Impurezze
≤0.00016 meq/g Acidity
≤0.5% Water
Residuo dopo evaporazione
≤0.005%
Indice di rifrazione
n20/D 1.344 (lit.)
P. ebollizione
81-82 °C (lit.)
Punto di fusione
−45 °C (lit.)
Temp. transizione
flash point 2 °C
Densità
0.786 g/mL at 25 °C (lit.)
applicazioni
environmental
food and beverages
industrial qc
pharmaceutical
Formato
neat
Condizioni di spedizione
ambient
Temperatura di conservazione
−20°C
Stringa SMILE
CC#N
InChI
1S/C2H3N/c1-2-3/h1H3
WEVYAHXRMPXWCK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
1 of 4
Questo articolo | 1.37134 | 1.13358 | 1.03725 |
|---|---|---|---|
| application(s) environmental | application(s) pharmaceutical | application(s) food and beverages | application(s) - |
| assay ≥90% (HPLC) | assay ≥99.5% (GC) | assay ≥99.8% (GC) | assay ≥99.9% (GC) |
| Quality Level 100, 200 | Quality Level 500 | Quality Level 200 | Quality Level 200 |
| grade for analytical purposes, for extraction, for synthesis, for cleaning, for preparative purposes | grade - | grade for chromatography, for preparative HPLC, for preparative liquid chromatography | grade - |
| form liquid | form liquid (colorless) | form liquid | form liquid |
| vapor pressure 72.8 mmHg ( 20 °C), 97 hPa ( 20 °C) | vapor pressure 72.8 mmHg ( 20 °C) | vapor pressure 72.8 mmHg ( 20 °C), 97 hPa ( 20 °C) | vapor pressure 72.8 mmHg ( 20 °C), 97 hPa ( 20 °C) |
Descrizione generale
Applicazioni
- Electroanalysis and Spectroelectrochemistry: Acetonitrile is widely used in electroanalysis and spectroelectrochemistry due to its excellent solvent properties and compatibility with a variety of electrodes. Recent research has demonstrated its use in the electroanalysis of nonaromatic explosives in the presence of dissolved oxygen. This application enhances the sensitivity and accuracy of detecting explosive compounds, which is critical for environmental monitoring and safety assessments (Holubowitch et al., Analytical Chemistry, 2020).
- Chromatographic Separation of Anthocyanins: Acetonitrile is a preferred solvent in high-performance liquid chromatography (HPLC) for the separation of anthocyanins and other phenolic compounds. Studies have explored the selectivity control of anthocyanin separation by replacing acetonitrile with methanol in the mobile phase, highlighting the solvent′s role in optimizing chromatographic conditions for improved resolution and detection sensitivity (Deineka et al., Journal of Analytical Chemistry, 2021).
- Peroxyoxalate Chemiluminescence Detection: Acetonitrile is used in peroxyoxalate chemiluminescence for the detection of α-amino acids. The solvent′s ability to form stable mixed solutions with water and ethyl acetate enhances the chemiluminescence reaction, allowing for sensitive and selective detection of amino acids in various samples. This application is essential for biochemical and clinical analyses (Kan et al., American Journal of Analytical Chemistry, 2020).
- Electrochemical Detection of DNA Modifications: Acetonitrile is utilized in electrochemical methods for detecting DNA modifications, such as 5-formyluracil. The solvent′s role in stabilizing the (2-benzimidazolyl) acetonitrile labeling reaction enhances the selectivity and sensitivity of detecting DNA modifications, which is crucial for genomic and biomedical research (Tang et al., Analytical Chemistry, 2021).
- Alternative Solvent in Chromatography: Research has investigated replacing acetonitrile with ethanol in reversed-phase HPLC for determining anthocyanins. This substitution aims to maintain chromatographic performance while reducing the environmental impact and costs associated with acetonitrile use. The findings demonstrate the feasibility of using ethanol as a greener alternative without compromising analytical quality (Deineka et al., Journal of Analytical Chemistry, 2023).
Risultati analitici
Identity (IR): conforms
Acidity: ≤ 0.00016 meq/g
Density (d 20 °C/ 4 °C): 0.782 - 0.783
Evaporation residue: ≤ 0.005 %
Water: ≤ 0.5 %
Note legali
Esclusione di responsabilità
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°C)
2.0 °C - closed cup
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica