If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/418/501/product-dating-information-06-25-mk.pdf
Wybierz wielkość
382,50 zł
382,50 zł
Cena katalogowa450,00 złZaoszczędź 15%Informacje o tej pozycji
Przejdź do
Nazwa produktu
Citric acid, Anhydrous, Pharmaceutical Secondary Standard; Certified Reference Material
InChI key
KRKNYBCHXYNGOX-UHFFFAOYSA-N
InChI
1S/C6H8O7/c7-3(8)1-6(13,5(11)12)2-4(9)10/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)
SMILES string
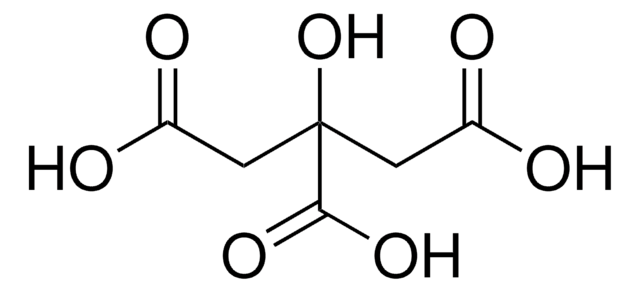
OC(=O)CC(O)(CC(O)=O)C(O)=O
grade
certified reference material
pharmaceutical secondary standard
agency
traceable to Ph. Eur. A1202000
traceable to USP 1134368
API family
citric acid
CofA
current certificate can be downloaded
expl. lim.
8 %, 65 °F
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
pKa
(1) 3.13, (2) 4.76, (3) 6.4
mp
153-159 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
Quality Level
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
1 of 4
Ta pozycja | 1134368 | A1202000 | 1.00241 |
|---|---|---|---|
| application(s) pharmaceutical (small molecule) | application(s) pharmaceutical (small molecule) | application(s) pharmaceutical (small molecule) | application(s) liquid formulation |
| technique(s) HPLC: suitable, gas chromatography (GC): suitable | technique(s) - | technique(s) - | technique(s) - |
| format neat | format neat | format neat | format - |
| Quality Level 300 | Quality Level - | Quality Level - | Quality Level 500 |
| grade certified reference material, pharmaceutical secondary standard | grade pharmaceutical primary standard | grade pharmaceutical primary standard | grade - |
| storage temp. 2-30°C | storage temp. - | storage temp. 2-8°C | storage temp. 2-30°C |
Analysis Note
Application
General description
Citric acid is a common ingredient in pharmaceutical formulations. It finds use in antacids and dentrifices because of the effervescent effect it generates upon combination with carbonates or bicarbonates. Citric acid may also be used as a buffering agent and it assists in the dispersion of suspensions in order to ensure the stability of the active ingredients and enhance the efficacy of antioxidants.[2]
Other Notes
comparable product
related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Klasa składowania
11 - Combustible Solids
wgk
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Protokoły
Science Slam panel: Leading gene therapy developers discuss commercialization challenges and the importance of robust process development plans.
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
Active Filters
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej