Polyethylene Glycol (PEG) Selection Guide
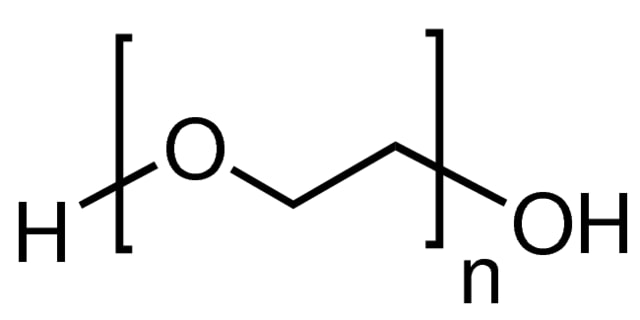
Poly(ethylene glycol) (PEG) structure
What is Polyethylene Glycol?
Poly(ethylene glycol) (PEG) is a synthetic, hydrophilic, biocompatible polymer with widespread use in biomedical and other applications. PEGs are synthesized using a ring-opening polymerization of ethylene oxide to produce a broad range of molecular weights and molecular weight distributions (polydispersity); however, discrete PEGs (dPEG® reagents) are synthesized with a single, specific molecular weight. PEGs can be synthesized in linear, branched, Y-shaped, or multi-arm geometries. PEGs can be activated by the replacement of the terminal hydroxyl end group with a variety of reactive functional end groups enabling crosslinking and conjugation chemistries.
Featured PEG Products
Response not successful: Received status code 500
How is Polyethylene Glycol used?
PEGs are non-toxic, FDA-approved, generally nonimmunogenic, and are frequently used in many biomedical applications including bioconjugation,1 drug delivery,2,3 surface functionalization,4 and tissue engineering.5 Bioconjugation with PEG (also known as PEGylation) is the covalent conjugation of drug targets such as peptides, proteins, or oligonucleotides with PEG for the optimization of pharmacokinetic properties.6 In drug delivery, PEGs can be used as linkers for antibody-drug conjugates (ADCs)7 or as a surface coating on nanoparticles to improve systemic drug delivery.6 PEG hydrogels are water-swollen, three-dimensional, polymer networks resistant to protein adhesion and biodegradation.8 PEG hydrogels are produced by crosslinking reactive PEG end groups and are commonly used in tissue engineering and drug delivery.
Functionality
| Polymer Architecture
|
Reactivity
| Molecular Weight
|
Common functional groups and their corresponding reactive groups are listed in the table below.
| Functional Groups | Reactive Groups | ||
|---|---|---|---|
| Primary Amine (–NH2) | NHS⧧ Ester Aldehyde Anhydride Epoxide | Isocyanate Sulfonyl Chloride Fluorobenzene Imidoester | Carbodiimide Acyl Azide Carbonate Fluorophenyl Ester |
| Thiol (–SH) | Maleimide Pyridyl disulfide | Haloacetyl Vinylsulfone | Iodoacetyl |
| Carboxyl (–COOH) | Amines | ||
| Carbonyl (–CHO) | Hydrazides | Alkoxyamines | |
References
To continue reading please sign in or create an account.
Don't Have An Account?