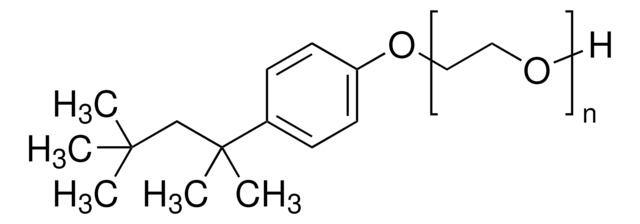
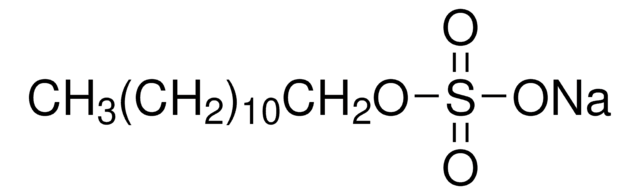Lysis & Protein Extraction
When purifying proteins for functional or structural studies, or for preparative processing and production, the first step is to disrupt the cells or tissue to gain access to the target proteins. Cell lysis and protein solubilization are key to effective analysis and efficient processing. The choice of extraction method can be enzymatic, chemical, mechanical or a combination.
Featured Categories
Protein extraction kits, cell lysis buffers, and reagents for solubilizing proteins from bacteria, yeast, and insect cultures, as well as plant and mammalian cell cultures and tissue samples.
Protease and phosphatase inhibitors and cocktails to prevent proteolysis and protein dephosphorylation, and to preserve the active state of proteins during cell lysis, protein extraction, and sample preparation.
Uncover proteins with precision: Explore kits for subcellular fractionation, protein enrichment, & extraction across diverse sample types in proteomics research.
Discover versatile biological detergents, surfactants for lysis, electrophoresis, WB, transfection, research applications. REACH-compliant options available.
Numerous methods are available for disrupting cells and preparing their contents for analysis. In general, gentle methods are employed when the sample consists of easily lysed cultured cells or blood cells, whereas more vigorous methods are employed for the disruption of more robust bacterial or plant cells, or mammalian cells embedded in connective tissue.
- Detergent-based lysis: Detergent-based lysis: Detergent lysis is a gentle method that can be used for mammalian cells, bacterial cells, yeasts, and plants. Cell suspensions are gently centrifuged and resuspended in lysis solution containing detergents which act to disrupt the cell membrane. The membranes are solubilized, lysing cells and liberating their contents. Detergents may need to be removed downstream if they interfere with analysis or production.
- Freeze-thaw lysis: This method is applicable to suspensions of mammalian or bacterial cells. The cell suspension is rapidly frozen using liquid nitrogen. The sample is then thawed and resuspended by pipetting or gentle vortexing in lysis buffer, repeating the process several times. Between cycles the sample is centrifuged, and the supernatant containing soluble protein is retained.
- Osmotic shock: This is a very gentle method that may be sufficient for the lysis of suspended mammalian or bacterial cells without the use of a detergent. The method, often combined with mechanical disruption, relies on changing from high to low osmotic medium, and is well-suited to applications in which the lysate is to be subsequently fractionated into subcellular components.
- Ultrasonication: This method of protein extraction is most frequently applied to cell suspensions. Cells are disrupted by high-frequency sound waves via a probe inserted in the sample. The sound waves generate a region of low pressure, causing disruption of the cell membranes.
- Mechanical methods: Proteins may be extracted from cells and tissues using various crude but effective "crushing and grinding" measures. For example, cell membranes may be disrupted by liquid shear forces using Dounce or Potter-Elvehjem homogenization. Tissues may be homogenized by chopping or mincing in chilled buffer using a Waring blender or Polytron® homogenizer. Tissues or cells may be frozen in liquid nitrogen and ground to a fine powder using a mortar and pestle with alumina or sand. Rapid agitation of cells with fine glass beads disrupts cell walls, effective for most Gram positive and Gram negative bacteria.
- Enzymatic digestion: Enzymatic digestion: Enzymatic methods are frequently used when extracting proteins from bacteria, yeast, or eukaryotic cells embedded in fibrous tissues where cell membranes are surrounded by a robust protective structure. Cell lysis enzymes or cocktails such as Lysozyme, Mutanolysin, MetaPolyzyme, Lysonase, and Pronase can be used in combination with tissue digestion enzymes (i.e. Collagenase, Chondroitinase, Hyaloronidase) to dissolve or disrupt cell walls, coats, capsules, capsids, or other structures not easily sheared by mechanical methods alone. Enzymatic digestion can be followed by homogenization, sonication, or vigorous vortexing in a lysis buffer.
Additionally, because endogenous proteases and phosphatases may be liberated upon cell disruption and degrade the target molecule, the sample should be protected during cell disruption and subsequent purification using protease and phosphatase inhibitors to avoid uncontrolled loss of target.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- This page lists nine frequently asked questions and answers about Benzonase® Nuclease.
- This page describes protein precipitation as an optional step in sample preparation for 2-D electrophoresis with Cytiva products.
- Enzymatic cell lysis and protoplast prep processes demystified, revealing complex enzymatic reactions during sample preparation.
- This article shows the use of BugBuster® and Benzonase® as protein purification tools to extract recombinant proteins from E. coli and to reduce the viscosity of the extract.
- The combination of BugBuster® and Lysonase™ reagents greatly enhances the release of soluble proteins from Gram-positive bacteria.
- See All (17)
Related Protocols
- Protocol for sample preparation for cell lysis and efficient protein extraction from cultured tissues and cells for subsequent Western blotting.
- TRI Reagent enables simultaneous DNA, RNA, and protein isolation with sample prep guidelines and troubleshooting.
- How to isolate proteins from inclusion bodies using Cytiva products.
- Detergent-free procedure is recommended for nuclear protein preparation to avoid interference with labeling efficiency of extracted proteins.
- Liberase™ TM Research Grade Protocol & Troubleshooting
- See All (13)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email [email protected]
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?