Bioink Selection for 3D Bioprinting
What Is 3D Bioprinting?
3D bioprinting enables the generation of precisely controlled 3D cell models and tissue constructs, by engineering anatomically-shaped substrates with tissue-like complexity. Due to the high degree of control on structure and composition, 3D bioprinting has the potential to solve many critical unmet needs in medical research, including applications in cosmetics testing, drug discovery, regenerative medicine, and functional organ replacement.1 Personalized models of disease can be created using patient-derived stem cells, such as induced pluripotent stem cells (iPS cells) or mesenchymal stem cells. Depending on the application, a range of materials, methods, and cells can be used to yield the desired tissue construct (Figure 1). For more in-depth information, including expert review articles on 3D Bioprinting, protocols, and related products, please explore our 3D Bioprinting Handbook.

Figure 1.3D Bioprinting of tissue and organs. Bioinks are created by combining cultured cells and various biocompatable materials. Bioinks can then be 3D bioprinted into functional tissue constructs for drug screening, disease modeling, and in vitro transplantation.
What Are Bioinks?
Bioinks contain living cells and biomaterials that mimic the extracellular matrix environment, supporting cell adhesion, proliferation, and differentiation after printing. In contrast to tradtional 3D printing materials, bioinks must have:
- Print temperatures that do not exceed physiological temperatures
- Mild cross-linking or gelation conditions
- Bioative components that are non-toxic and able to be modified by the cells after printing
Bioinks for Extrusion-based Printing
Cell-encapsulating hydrogels are used in 3D bioprinting to create living tissue structures by forming multicellular bioprinting building blocks. Cell encapsulation allows for precise control over cell attachment and the spatial distribution of the cells and biomolecules within the scaffold, in comparison to other methods and materials.1 Combining multiple cell types and growth factors in a prescribed pattern allows for the generation of highly-complex tissue constructs.3 In addition to biocompatibility, bioprinting materials used for cellular encapsulation must feaure high water content and porosity, allowing encapsulated cells to receive nutrients and remove waste.1 As water-swollen, porous networks, hydrogels are ideal materials for cell-encapsulation, tissue engineering, and 3D bioprinting applications. Hydrogels for 3D bioprinting must also feature tunable substrate stiffness and allow for network remodeling post-printing, so cells can spread, migrate, proliferate, and interact.9 While a wide variety of materials are used for bioinks, the most popular materials include gelatin methacrylol (GelMA), collagen, poly(ethylene glycol) (PEG), Pluronic®, alginate, and decellularized extracellular matrix (ECM)-based materials (Table 1).
Featured Bioink Material
Gelatin MethacryloylGelatin methacryloyl (GelMA) can be used to form crosslinked hydrogels for tissue engineering and 3D printing. GelMA-based bioinks feature excellent cytocompatibility, tunable substrate stiffness, improved printability, and rapid crosslinking with exposure to UV or visible light (depending on the identity of the photoinitiator)11. GelMA has been used in endothelial cell morphogenesis, cardiomyocytes, epidermal tissue, injectable tissue constructs, bone differentiation, and cartilage regeneration. Gelatin methacryloyl has also been used in microspheres and hydrogels for drug delivery applications.
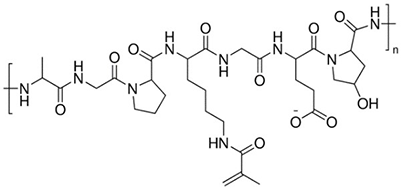
Figure 2.Gelatin Methacryloyl
Acellular Materials
In addition to bioinks, acellular materials are also used in 3D bioprinted structures.2 Acellular materials typically provide structural support for tissue constructs and when utilized with bioinks, can generate functional, bioprinted tissues. Acellular materials are porous structures that recapitulate both mechanical and biochemical properties of the native extracellular matrix (ECM)4. Porosity enables cell migration, tissue growth, vascular formation, and cell viability within these structural constructs.6 In addition, acellular materials must also have the necessary surface chemistry for cell attachment, proliferation, and differentiation.5 Popular acellular materials include: collagen, fibrin, chitosan, nanocellulose, poly(lactic acid) (PLA), polycaprolactone (PCL), hydroxyapatite (HA), and β-tricalcium phosphate (β-TCP) (Table 1).
Bioink Material Building Blocks | |||
|---|---|---|---|
| Bioink Material | Overview | Advantage | Disadvantage |
| Agarose | Polysaccharide extracted from seaweed | Non-toxic crosslinking High stability | Not degradable; Poor cell adhesion |
| Alginate | Naturally-derived biopolymer from brown algae | Mild crosslinking conditions (Ca2+) Rapid gelation High biocompatibility | Slow degradation kinetics; Poor cell adhesion |
| Chitosan | Polysaccharide obtained from the outer skeleton of shellfish (e.g. shrimp). Non-animal derived chitosan can be obtained from fungal fermentation. | High biocompatibility Antibacterial properties | Slow gelation rate |
| Collagen | Primary structural protein found in skin and other connective tissues | High biological relevance | Acid-soluble |
| Decellularized ECM | Isolated extracellular matrix of a tissue from inhabiting native cells | High biological relevance Tissue-specific High cell survival | Undefined and inconsistent; Loss of native ECM organization; Low stability |
| Fibrin/Fibrinogen | Insoluble protein formed during blood clotting | High biological relevance Rapid gelation | Limited printability |
| Gelatin | Protein substance derived from partial hydrolysis of collagen | High biocompatibility High water solubility Thermally reversible gelation | Poor shape fidelity; Limited rigidity |
| Graphene | Carbon-based material that can be viewed as a one atom thick sheet of graphite | Flexible Electrically-conductive | Low biological relevance |
| Hyaluronic Acid (HA) | Non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. | Fast gelation Promotes cell proliferation | Poor stability |
| Hydroxyapatite | Naturally-occurring mineral form of calcium apatite found in teeth and bones | High strength and rigidity | Low printability; Limited tissue specificity |
| PCL/PLA/PLGA | Biodegradable, thermoplastic polymers and/or copolymers | High strength and rigidity | Low cell adhesion and proliferation |
| Pluronic® F127 | Poly(ethylene oxide) and poly(propylene oxide) block copolymer | Printable at room temperatures Shear thinning material | Not suitable for long-term cell culture |
What 3D Bioprinting Method Should Be Used?
Depending on the type of ink (bioink or acellular materials) selected and complexity of the final tissue construct, different 3D printing methods can be used (Figure 1). Advantages and disadvantages of common methods can be found in the table below (Table 2).
| Printing method | Advantages | Disadvantages | Cell compatible? |
|---|---|---|---|
| Extrusion-based |
|
| Yes |
| Inkjet-based |
|
| Yes |
| Stereolithography (SLA) |
|
| Yes |
| Laser-based |
|
| Yes |
| Fused-deposition modeling (FDM) |
|
| No |
| Selective laser sintering (SLS) |
|
| No |
In addition to ink type, the bioprinting method can also be dictated by the end application of the printed construct (Table 3).
Conclusion
3D bioprinting allows for the spatially-controlled placement of cells in a defined 3D microenvironment. Bioinks are formed by combining cells and various biocompatible materials, which are subsequently printed in specific shapes to generate tissue-like, 3D structures. Combing our expertise in materials science and cell biology, we offer a variety of solutions to simplify the 3D bioprinting workflow.
References
To continue reading please sign in or create an account.
Don't Have An Account?